SAMI
Published:2020-05-01 07:05:15 BdST
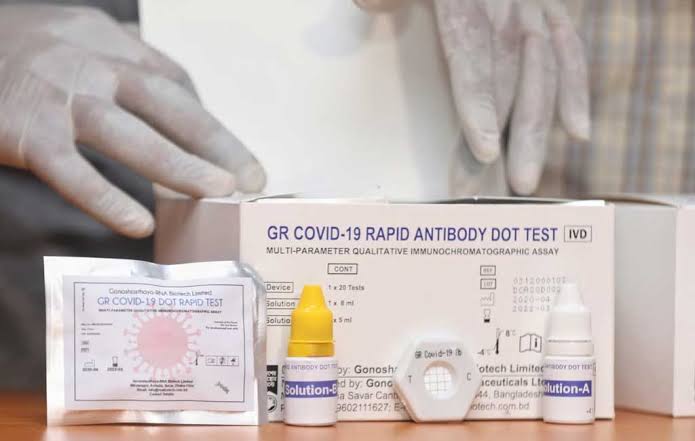
Gonoshasthaya's Covid-19 testing kit permitted for trial
The Directorate General of Drug Administration has given permission to Gonoshasthaya Kendra to carry out testing for Covid-19 with the kit they invented - GR Covid-19 Dot Blot - on a trial basis.
Confirming the matter to media, Dr. Zafrullah Chowdhury, founder and trustee of Gonoshasthaya Kendra, said that the trial will begin at Bangabandhu Sheikh Mujib Medical University Hospital (BSMMU) from Saturday.
"We requested BSMMU to conduct the trials before. But they told us that they cannot do it without permission from the drug administration," he said.
Now that Gonoshasthaya Kendra has received permission from the drug administration, BSMMU will begin the trials.
Gonoshasthaya Kendra received the letter from the drug administration today.
When asked what was written in the letter, Dr Zafrullah Chowdhury said that the letter said trials can be done at BSMMU or at the icddr,b. It also requested BSMMU and icddr,b to conduct the trials to test the effectiveness of GR Covid-19 Dot Blot.
"The approval process has advanced because of this. We wanted this two-line letter. But they wanted to appoint a middle-man instead. That was our objection," Dr Zafrullah added.
The DG of the Directorate General of Drug Administration Major General Mahbubur Rahman said, "Gonoshasthaya is free to conduct trials at BSMMU or icddr,b. We will check the sensitivity, specifications and effectiveness after the trial, and take a decision on approval."
Unauthorized use or reproduction of The Finance Today content for commercial purposes is strictly prohibited.