SAMI
Published:2020-05-11 04:04:33 BdST
Gonoshasthaya test kits could undergo validation from Monday
The comparative effectiveness test of Gonoshasthaya Kendra's Covid-19 test kits which was supposed to start on Sunday could begin on Monday, founder and trustee Dr Zafrullah Chowdhury said.
"The Bangabandhu Sheikh Mujib Medical University Hospital [BSMMU] informed us about the development yesterday and they also said that they had issued a letter."
Asked about the letter, Coordinator of the GR Covid-19 Dot Blot Project Dr Muhibullah Khandaker said, "We are yet to receive it."
Earlier on May 10, the BSMMU formed a six-member committee to conduct the performance study of the Covid-19 rapid testing kits developed by Gonoshasthaya.
"We came to know about the letter while talking to one of the evaluation committee members over the phone," said Dr Muhib Ullah.
However, the former chairman of the virology department at BSMMU Dr Shahina Tabassum, who is also the head of the committee, could not be reached over the phone for comment till the filing of this report.
Asked about the delay in performance study, Dr Zafrullah said, "It is their internal issue. But I am still hopeful that once the test begins, it will finish within two-three days."
Gonoshasthaya wants to apply to the Directorate General of Drug Administration for registration of the test kits on May 17.
Earlier, Dr Zafrullah claimed that some countries had been trying to develop test kits by copying their model.
"India has already taken a big leap in this direction and it has set the price for its kit at ₹500 only. We must go to production as soon as possible," he added.
On April 30, the drug administration directorate gave approval to Gonoshasthaya to get its Covid-19 kits tested.
Director General of Drug Administration Major General Md Mahbubur Rahman said they will evaluate the results of the performance trial prior to the registration.
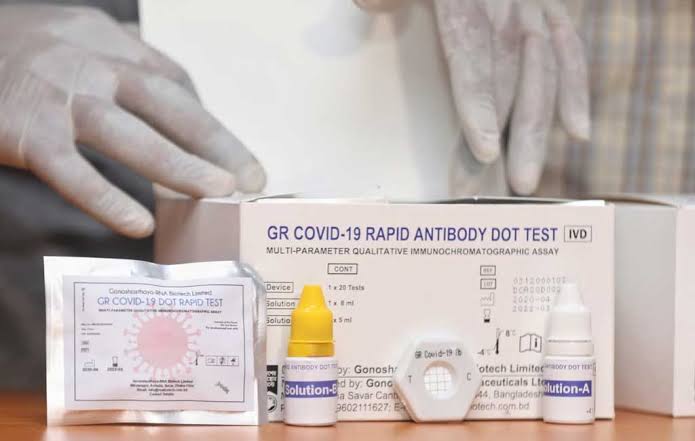
Gonoshasthaya-RNA Biotech Limited, a concern of Gonoshasthaya Kendra in Bangladesh, said last month that it had developed a method named "Gonoshasthaya Rapid Dot Blot" to test Covid-19.
The drug directorate earlier refused to accept the kit, but later permitted the kit's performance trial at the BSMMU.
Unauthorized use or reproduction of The Finance Today content for commercial purposes is strictly prohibited.