SAMI
Published:2020-05-09 04:26:37 BdST
Gonoshasthaya kit performance trial likely to begin Sunday
The Bangabandhu Sheikh Mujib Medical University (BSMMU) will begin the performance trial of the Covid-19 testing kit developed by Gonoshasthaya Kendra on Sunday, said Dr Zafrullah Chowdhury.
Though Dr Zafrullah, founder and trustee of the Gonoshasthaya Kendra, is hopeful about the performance trial to be completed within two to three days, BSMMU Vice-Chancellor Prof Kanak Kanti Barua did not specify the completion date.
"It is a confidential matter," said Prof Barua, adding that there are many steps prior to the performance trial.
On Thursday, the Bangladesh Medical Research Council (BMRC) issued the ethical clearance to the Gonoshasthaya Kendra kit.
After the go-ahead from the BMRC, the Gonoshasthaya kit will now have to pass BSMMU performance trial before applying for registration from the Directorate General of Drug Administration.
Now the BSMMU says it will begin the trial once the earlier formed six-member technical committee makes and approves the protocol for the kit.
BSMMU VC Prof Barua said the committee led by the medical university virology department Prof Dr Shahina Tabassum will evaluate the effectiveness of the kit.
"We hope the BSMMU will not make any delay considering the urgency for the kit," said Dr Zafrullah.
The Gonoshasthaya Kendra hopes to apply for registration from the drug directorate on May 17.
Zafrullah claimed that some countries had been already trying to develop test kits copying them.
"India has already taken a big leap on this and they have set the price for their kit at 500 rupees only. We must go for production as soon as possible," he added.
Dr Bijon Kumar Sil, who led the team in developing the kit, claimed earlier that they had internally validated the kit and found it to be successful.
On April 30, the drug directorate gave approval to determine the effectiveness of the G-Rapid Dot Blot kit.
"The kit will be tested either at the BSMMU or at the ICDDR,B, or at both institutes," said the directorate Director General Major General Md Mahbubur Rahman.
He said they will evaluate the results of the performance trial prior to the registration.
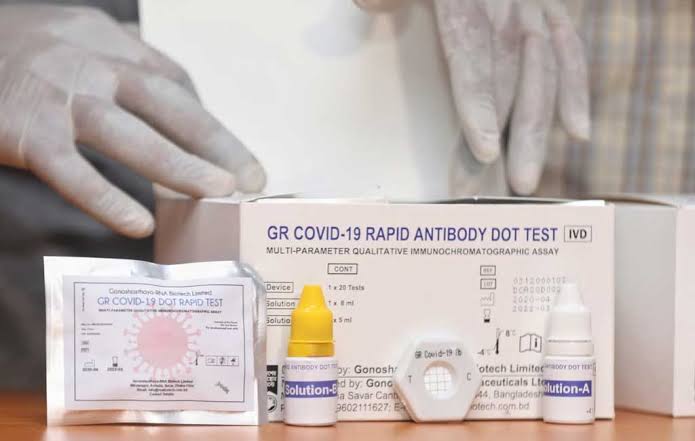
Gonoshasthaya-RNA Biotech Limited, a concern of Gonoshasthaya Kendra in Bangladesh, said last month that it had developed a method named Rapid Dot Blot to test Covid-19.
The drug directorate earlier refused to accept the kit but later permitted the kit's performance trial at the BSMMU.
Unauthorized use or reproduction of The Finance Today content for commercial purposes is strictly prohibited.